OUR OFFERING
Keys to Formulation Development
Supported by a Talented Specialized R&D Center
Genuone Sciences operates centralized R&D and Process Technology Research Center hubs focused on contract development and manufacturing (CDMO) with robust support from six teams. Our R&D division is built on a system optimized to perform government projects, mid- to long-term R&D strategies as well as a variety of other research missions. Our excellent drug delivery system (DDS) technology allows us to research and produce the full scope of formulations to meet customer specifications. Nearly 9 out of every 10 (89%) of our R&D employees hold masters or doctorate degrees in the field. Many of our professional researchers have deep experience in specific formulations, including principal investigator (PI) level researchers.
- NEO-DDS
TECH - Controlled PK-based DDS technology
Incrementally Modified Drug (IMD) development technology
New formulation development technology
- GLOBAL & OPEN
INNOVATION - Collaborative industry-academic research with domestic and international organizations
Collaborative research with domestic and international research institutes
Accessible & open development networking
- CONVERGENCE
TECH - Multifunctional DDS technology
In Vitro In Vivo Correlation (IVIVC)
Fusion tech with cosmeceuticals
We are increasing R&D capabilities and expanding self-developed new drugs to strengthen global competitiveness. Making strategic use of South Korea's advanced technology and creativity, we are leading the nation’s pharmaceutical industry with cutting-edge research equipment that introduces original formulation manufacturing technology and innovative drug delivery solutions.
Successes include our newly developed Lidcap® (lliquid-filled hard gelatin capsules), a formulation manufactured with our proprietary technology that fills hard capsules with liquids to treat diseases more effectively. We developed improved skincare formulations with fusion technology that combines advanced cosmetic technology with pharmaceutical efficacy. In addition to developing generic medicines for several branded synthetic drugs, we have secured and continue to accumulate advanced technology that pioneers novel therapeutic options for existing drugs or creates new incrementally modified drugs by changing administration or dosage.
We are also working to enhance drug stabilities. For example, we are improving drug absorption by boosting drug solubility using nanoparticles or self-micro emulsifying drug delivery systems to enhance solubility of poorly water-soluble drugs, sustained releases and formulation changes.
Our research center has applied for and registered many patents in the R&D stage of new products. We are also building a system to discover and evaluate various materials with potential for new drugs.
We have various techniques for screening and judiciously selected animal models that lay the foundation for efficacy evaluation of new anti-inflammatory drug development. Using our sophisticated formulation development processes and analysis experience, we routinely review external patented technologies and raw materials in order to evaluate their usefulness in expanding our formulations business.
At the same time, we are exploring wider therapeutic avenues and partnerships to accelerate development of medicines for more efficient treatment of diseases. Currently, for example, we are collaborating with domestic and international universities and government-funded research institutes to develop atopic dermatitis (eczema) medicine and antibiotics as well as with other ingredients, such as natural substances and synthetic compounds. We also launched an Open Innovation Lab to explore multiple projects, develop new technologies and discover new materials. And we are developing high-value-added technologies and investing in developing biopharmaceuticals to lead the future of the pharmaceutical industry.
Lidcap® (liquid-filled hard gelatin capsules) Manufactured by Genuone Sciences, with South Korea’s first and only proprietary production technology in pharmaceuticals
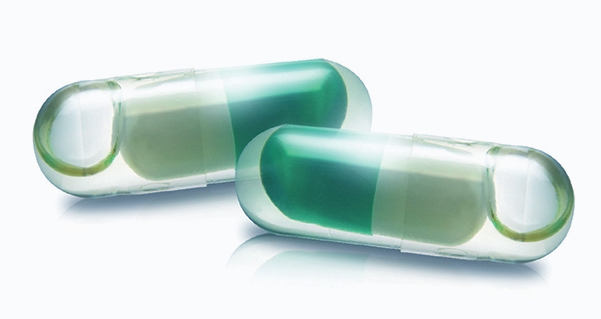
- Technology used to fill hard capsules with liquid or semi-solid contents
- Formulation stability enhanced with sealing to prevent leakage
- Achieves higher formulation stability than soft capsules
- Accelerates onset of drug effect
- Uses plant-based capsules
- Available in various types of release, including capsule or tablet within capsule
- Reduced size of dosage form possible with heated filling
